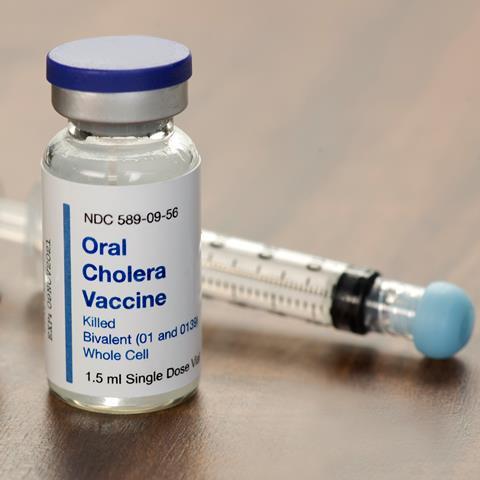
The World Health Organization (WHO) has declared a worldwide shortage of the oral cholera vaccine (OCV) amid an upsurge in cases around the globe.
The available stockpile has reduced significantly as the number of doses approved for shipment to outbreak areas has grown rapidly in recent months.
The WHO recorded 40,900 cholera cases and 775 deaths in January alone from 17 countries across Africa, the eastern Mediterranean, Central America and South-East Asia. Currently, the most severely affected countries are predominantly in Africa and include the Democratic Republic of the Congo, Ethiopia, Somalia, Sudan, Zambia and Zimbabwe. Elsewhere in the world, Haiti and Syria are also badly affected.
Global production capacity of the OCV in 2024 is expected to be close to 50 million doses, in contrast to the 36 million vaccines produced in 2023 . The International Coordinating Group on Vaccine Provision (ICG), which manages the stockpile, said production capacity of only 50 million doses this year would likely be inadequate to manage the outbreak.
The shortages are still likely despite the ICG cutting its recommended OCV dosing in 2022 from two to one. Even at one dose, the OCV, developed by the International Vaccine Institute (IVI), can still provide substantial protection against cholera for a number of years.
Currently the only manufacturer that produces the vaccine is EuBiologics, with new manufacturers not expected to join the market before 2025.
EuBiologics is simplifying the production of its original vaccine, Euvichol-Plus, in order to keep up with the growing demand. The new formulation, called Euvichol-S, contains two strains of inactivated V. cholorae bacteria rather than five and drops the heat inactivation step.
According to a press release from IVI, the reformulated vaccine will expand production capacity by 38% to about 52 million doses annually. A phase III trial conducted in Nepal in 2022 showed the simplified version to be as effective as the original.
IVI is also developing an injectable conjugate vaccine which could offer better and longer lasting protection in children, for whom the oral vaccine is least effective. It could also be used to immunise infants, who are not currently licensed to receive the oral vaccine.
“The same urgency and innovation that we saw for COVID-19 must be applied for cholera,” said the WHO.
Further reading:
Oral Cholera Vaccine Efficacy and Effectiveness
World Health Organisation: Multi-country outbreak of cholera, external situation report
International Vaccine Institute: Euvichol-S, simplified formulation of oral cholera vaccine, licensed by Korean regulatory agency