The first approval of a therapy using the Nobel Prize-winning gene-editing tool CRISPR/Cas9 has been made in the UK by the MHRA. The gene therapy, called Casgevy, will treat patients with sickle-cell disease and transfusion-dependent β-thalassemia in patients aged 12 years and over.
Casgevy uses the CRISPR/Cas9 enzyme to target, and substitute, faulty genes in the patient’s bone marrow to rectify stem cell production so that the body can make functioning haemoglobin.
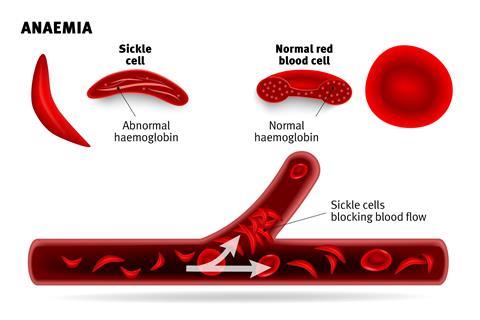
Sickle-cell disease and β-thalassemia are genetic conditions caused by errors in the genes that control the production of haemoglobin. In people with sickle-cell disease, this can cause red blood cells to malform from their normal, flat, doughnut shape (see picture) and presents with symptoms that including pain episodes broadly called ‘sickle cell crises’. These include several acute conditions such as the vaso-occlusive crisis, aplastic crisis, splenic sequestration crisis, hyperhaemolytic crisis, hepatic crisis – and increase the risk of serious infections and anaemia.
In people with β-thalassaemia, it can lead to severe anaemia due to the body being unable to generate sufficient β-haemoglobin necessitating regular blood transfusion to boost levels red blood cells.
Prior to the UK regulator’s approval, a bone marrow transplant – which must come from a closely matched donor and carries the risk of rejection – was the only permanent treatment option available in both conditions.
In a clinical trial for sickle-cell disease involving 45 patients, 28 of these experienced relief from severe sickle-cell crises for at least 12 months following treatment with Casgevy. In a clinical trial for transfusion-dependent β-thalassemia involving 54 patients, 39 of these did not need a blood transfusion for at least 12 months following the treatment.
“Sickle cell disorder is an incredibly debilitating condition, causing significant pain for the people who live with it and potentially leading to early mortality,” said John James OBE, Chief Executive of the Sickle Cell Society.
“There are limited medicines currently available to patients, so I welcome today’s news that a new treatment has been judged safe and effective, which has the potential to significantly improve the quality of life for so many.”
Further reading:
Regulatory Rapporteur: Novel gene editing method ‘roughly threefold’ better: https://www.regulatoryrapporteur.org/industry-news/novel-gene-editing-method-roughly-threefold-better/292.article
Regulatory Rapporteur May 2023: How gene editing technologies could change more than genes: https://www.regulatoryrapporteur.org/pharmaceuticals/g-e-t-ting-there-how-gene-editing-technologies-could-change-more-than-genes/276.article